Our desire for rejuvenation is almost ubiquitous in our societies. If the “young” physical aspect is one of the objectives, another possibility is to stop the progressive deterioration of cellular mechanisms, vectors of diseases, chronic, neurodegenerative, etc. In addition, the world population is aging. According to the United Nations, one in six people in the world will be older than 65 (16 percent) by 2050, compared to one in 11 in 2019 (9%). Recently, researchers have developed a new method to reverse the aging of human cells by 30 years, revolutionizing regenerative medicine.
the aging is a continuous and gradual process of natural weathering that begins early in adulthood. During early middle age, many bodily functions begin to decline gradually. From a biological perspective, therefore, aging is the product of the accumulation of a wide range of molecular and cellular damage over time. These lead to a progressive deterioration of physical and mental capacities, an increase in the risk of disease and eventual death. These changes are not linear or regular and are not closely related to the number of years. But even if it’s unavoidable, aging can be affected.
This is why the regenerative medicine offers high expectations. The latter is aimed at repairing, replacing or regenerating faulty genes, cells or organs to restore normal functioning. It therefore has the potential to undo age-related changes. The treatments consist of grafting on the patient, in the damaged area, restoring cells. Once nestled in (or near) the target organ, these do the work themselves, restoring healthy tissue. These repair cells are stem cells. In simple terms, they are unspecialized – undifferentiated – cells capable of infinite self-renewal and, depending on the environment they are in, can give rise to the various constituent cells of the tissue.
The technique to obtain these cells is a process in which somatic cells are converted into induced pluripotent stem cells (iPSC). It consists of taking practically every cell of an adult and genetically reprogramming it to make it pluripotent, that is, capable of infinitely multiplying and differentiating into all the types of cells that make up an adult organism – such as an embryonic stem cell.
Unfortunately, after the numerous steps required for their reprogramming, these iPSC cells lose some of their specific functions acquired with age. They often resemble fetal cells more than mature adult cells. Recently, a team of researchers at the Babraham Institute in Cambridge developed a method to reprogram cells to become biologically younger while regaining specialized cellular function. The study was published in the journal eLife†
Go back in time to the “right moment”
To preserve the specific properties of the cells while allowing them to be rejuvenated, the researchers rely on the work of Shinya Yamanaka, who in 2007 was the first scientist to demonstrate the ability to convert normal cells into stem cells. This process takes about 50 days using four key molecules called the “Yamanaka factors.” This technical ability earned him the Nobel Prize in Physiology and Medicine in 2012. In addition, recent work has shown that the epigenome — any modifications of a cell that alter the expression of genes without altering the underlying DNA sequence — is already rejuvenated by the first stage of reprogramming (phase maturation). This suggests that complete reprogramming of iPSC is not required to reverse somatic cell aging.
Accordingly, the researchers used dermal fibroblasts from middle-aged donors to figure out when to stop the reprogramming process. They first exposed them to Yamanaka factors and found that the cells temporarily lost their fibroblast identity and regained it after just 13 days. This may be due to epigenetic memory at the level of activators and/or to the sustained expression of certain fibroblast genes. This new method is called “transient reprogramming of the maturation phase”.
Aging, a question of chronological or biological age?
As Dr Diljeet Gill explains in a communicatedpostdoctoral researcher in Wolf Reik’s laboratory at the Institute (who carried out the work as a doctoral student): Our understanding of aging at the molecular level has improved over the past decade, leading to techniques that allow researchers to measure age-related biological changes in human cells. We were able to apply this to our experiment to determine the degree of reprogramming of our new method. †

 100vw, 800px”/>
</picture>
</noscript><figcaption id=) dr. Diljeet Gill during the experiments. © Babraham Institute
dr. Diljeet Gill during the experiments. © Babraham InstituteTo check whether the regeneration process had been successful, they examined on the one hand what is called the epigenetic clock; the transcriptome on the other. The latter corresponds to all messenger RNA molecules of a cell, replicas of the genes that are active in a cell. For its part, the epigenetic clock is a mathematical model that predicts age by measuring DNA methylation levels at different sites in the genome.
You should know that DNA methylation is a process where methyl groups are added to the DNA molecule, which can change the function of a gene without changing the underlying DNA sequence. This DNA methylation is essential for healthy cell growth and development and is influenced by lifestyle and environmental factors. Epigenetic clocks can therefore be used to estimate the biological age of a tissue, cell type or organ, by comparing the “DNA methylation age” (or biological age) with the chronological age, in different tissues. Using these two measurements, the reprogrammed cells matched the profile of cells 30 years younger than the reference data sets.
Implications for regenerative medicine
As a result, the analysis showed that the cells had found characteristic markers of skin cells, specifically by observing the production of collagen in the reprogrammed cells. Fibroblasts produce collagen. This molecule is present in bones, tendons and ligaments and helps structure tissues and heal wounds. The researchers found greater collagen production by the rejuvenated fibroblasts than by the control cells (which had not undergone the reprogramming process).
 100vw, 900px”/>
</picture>
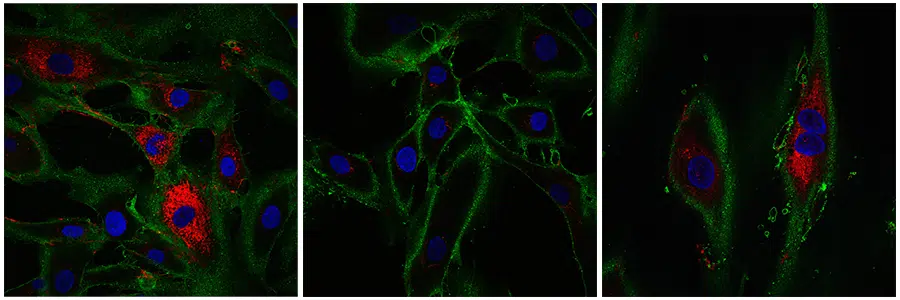
</noscript><figcaption id=) Left fibroblasts from a 20-22 year old subject. In the middle, aged fibroblasts that have not undergone reprogramming. On the right, reprogrammed cells. Collagen is shown in red. © Gill et al., 2022
Left fibroblasts from a 20-22 year old subject. In the middle, aged fibroblasts that have not undergone reprogramming. On the right, reprogrammed cells. Collagen is shown in red. © Gill et al., 2022Moreover, live, the fibroblasts move to the areas to be repaired. The researchers then tested this ability in partially rejuvenated cells. To do this, they cut into a layer of cells, like a cut in the skin. They found that their treated fibroblasts moved through space faster than older cells. The researchers point out that this is a promising sign for the future ability to create cells that can better heal wounds.
Finally, the analysis of the above-mentioned transcriptome revealed signs of rejuvenation at the level of two specific genes involved in age-related diseases and symptoms: the APBA2 gene, associated withAlzheimer, and the MAF gene, which plays a role in cataract development. Professor Wolf Reik, who leads the study, says: “ This work has very interesting implications. Ultimately, we can identify genes that rejuvenate without reprogramming, and specifically target those genes that reduce the effects of aging †
Even if the mechanism behind temporal reprogramming isn’t fully understood, scientists believe that key regions of the genome involved in forming cellular identity could escape the reprogramming process. Gill concludes: Our results represent a major step forward in our understanding of cellular reprogramming. We have proven that cells can be rejuvenated without losing function and that rejuvenation attempts to restore certain functions of old cells. The fact that we have also observed a reversal of aging indicators in disease-related genes is particularly promising for the future of this work. †



